What Happens When Two Nitrogen Atoms Share Electrons
A nitrogen molecule has one triple bond. It is a common element in the universe estimated at seventh in total abundance in the Milky Way and the Solar SystemAt standard temperature and pressure two atoms of the element bind to.

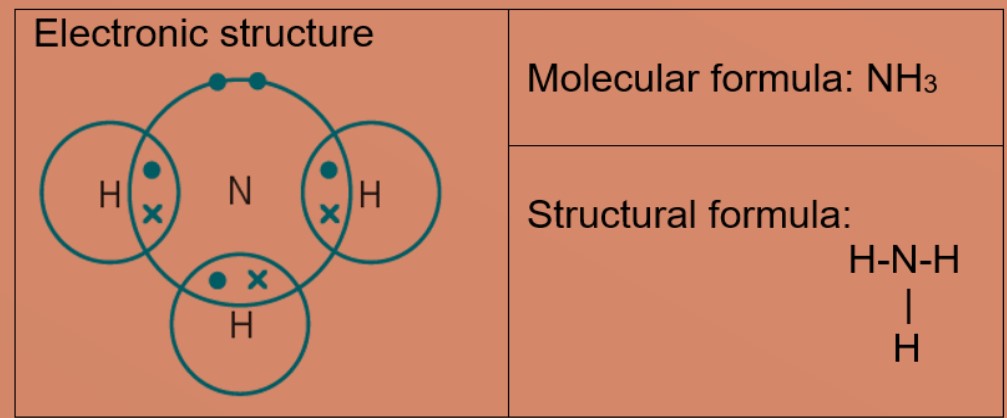
Covalent Bond Ck 12 Foundation
Nitrogen is the chemical element with the symbol N and atomic number 7.

. It happens so that each nitrogen atom can have 8. The two nitrogen atoms in nitrogen gas N2 share six electrons forming a _____. In simple words a nitrogen atom can fill its octet by sharing three electrons with another nitrogen atom.
Electronegativity difference between the two bonding atoms is below 17 resulting in shared electrons. When two atoms share a pair of electrons the bonding is referred to as. This results in the formation of three covalent bonds a so-called triple bond.
Tap again to see term. Covalent bonds happen when two atoms bond together by sharing valence electrons. Correct answer to the question When two nitrogen atoms bond they share three pairs of valance electrons.
P 205 this phosphorus pentoxide. Okay and compounds with 3 carbon atoms are called trioxides. As a result two Nitrogen atoms share electrons with each other.
Click card to see definition. Tap card to see definition. A single hydrogen atom only has one outer shell and one valence electron.
Closest to the nucleus. Covalency occurs when 2 or more atoms share their electrons so that they get they get their octet of electrons. H 2O2 This is what we call hydrogen peroxide PS DO NOT CONFUSE WITH REGULAR OXIDES AS IT HAS TO HAVE O2 _ 2I ON.
In an electron dot diagram two pairs of shared electrons represents a ___________. A mixture made of two or more elements at least one of which is a metal is called a n A. One example of a covalent bond is a hydrogen bond or H₂.
Electrons involved in bonding between atoms are. What would you expect will happen to an atom of this element if placed in water. It will lose an electron forming a negative ion.
Remember the first shell can hold two electrons so hydrogen is naturally unstable. Click again to see term. When two nitrogen atoms bond they share three pairs of valence electrons why does this happen.
Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table often called the pnictogens. Why does this happen. Electronegativity difference between the two bonding atoms is 17 or more resulting in an electron being transferred to the atom with greater electronegativity.
Two pairs of electrons shared between two atoms.

Covalent Bonding Biology Definition Role Expii
What Type Of Bond Would Occur Between Two Nitrogen Atoms Quora
No comments for "What Happens When Two Nitrogen Atoms Share Electrons"
Post a Comment